The dengue vaccine – an open letter to the MOH
(image credit: the MalayMail online)
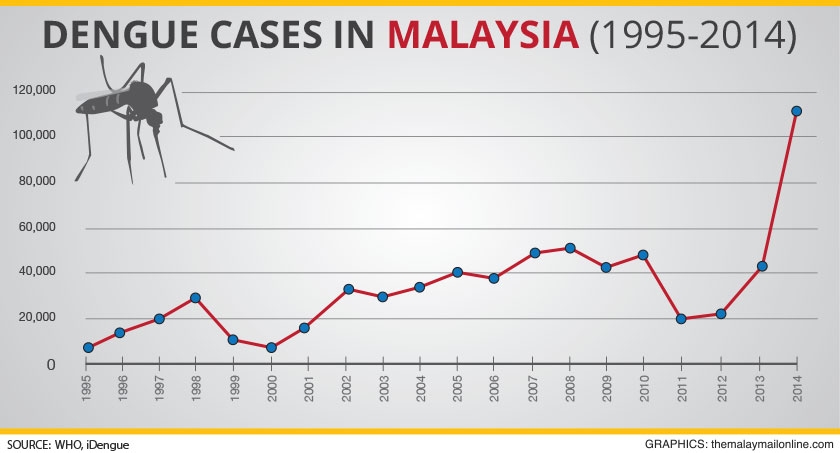
There’s no doubt about it – the statistics are grim. Dengue has seen a steep rise in 2014 and even higher numbers were seen in 2015. Early on in 2016, we have to brace ourselves for a spike due to the El Nino effect
The Star reported the very grim data:
According to the ministry, 336 people – an average of 28 a month – died from dengue last year compared to 215 in 2014, a rise of 56.3%.
There was also an increase of 11.2% in the number of dengue cases throughout last year, up from 108,698 in 2014 to 120,836 cases.
That’s 333 cases a month or 110 cases each day!
The ministry is now cautioning people to brace for an equally bad, if not worse, year ahead.
Just imagine, almost one person dying of dengue every day and over 100 cases every day last year, and the numbers are expected to worsen.
We all are aware of the importance of controlling the vector, the Aedes mosquito. The focus on this should not be lost but clearly we are losing the battle against dengue if we are only relying on this. Perhaps the authorities should double down on the public health measures and really impose stiff fines on those caught breeding Aedes but honestly do you think this will be enough given the current track record?
Many are aware that for the first time, there is now a vaccine available for the prevention of dengue produced by Sanofi, called Dengvaxia®
Dengvaxia®, a live attenuated tetravalent chimeric vaccine, is the first vaccine licensed for the prevention of dengue in the world. Sanofi Pasteur’s vaccine is a product of over two decades of scientific innovation as well as 25 clinical studies in 15 countries around the world. Approved in Mexico (December 9, 2015) and the Philippines (December 22, 2015) and regulatory approvals are expected in other countries where dengue is a public health priority.
Apart from Mexico and the Philippines, the Sanofi vaccine has also been approved in Brazil.
In Malaysia, it has been reported that we are “still studying” the vaccine data.
Actually we don’t know what is meant by “still studying” since the global clinical trials have been done including SEA where Malaysian patients participated, as well as in South America. The data has been published in reputable journals like the New England Journal of Medicine (Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease N Engl J Med. 2015 Sep 24;373(13):1195-206).
In the first reports from the trials, vaccine efficacy was 56.5% in the Asian study and 64.7% in the Latin American study in patients who received at least one injection of the vaccine. Efficacy varied by serotype. In both trials vaccine reduced by about 80% the number of severe dengue cases. Pooled efficacy and integrated safety analyses from the 25-month Phase III efficacy studies and the ongoing long-term studies, respectively, published in The New England Journal of Medicine (July 2015) affirmed the vaccine’s consistent efficacy and longer-term safety profile in study population 9-16 years of age. In the pooled efficacy analysis in this age group, Dengvaxia® was shown to reduce dengue due to all four serotypes in two-thirds of the participants and prevent 8 out of 10 hospitalizations and up to 93% of severe dengue cases.
So it clearly is not a perfect vaccine (is there any?) but the long term data shows it is safe. Any dengue after vaccination is not going to be worse, and it will reduce hospitalisation and severe dengue in those aged 9 years and above by up to about 80-90% overall.
If we are going to wait for a better vaccine we have to wait many more years and in the meantime many more will die or be hospitalised with severe dengue.
We can understand the hesitancy of the authorities in approving the vaccine given the concerns about the effectiveness in Malaysia – whether it will work well given the prevalent serotypes and at the end of the day will it reduce dengue deaths and hospitalisation.
So what we suggest to the MOH is that is we are really serious about tackling dengue is to not only talk about “studying” the vaccine but to really study it further by taking it to the field where we can get more data.
One way is to conduct pilot studies in dengue hot spots in Malaysia. This is what we believe the MOH should do and do quickly.
I can understand if funding is going to be an issue especially as the MOH budget has been slashed further this year. But perhaps think out of the box. Pilot studies need not be funded by MOH alone. You could rope in funding from public and corporate sponsorship and given the gains industries would see from reducing lost productive man hours due to workers falling ill from dengue it could well be a win win project. MOH can negotiate with Sanofi for a good price and both parties should compromise. Conduct the pilot studies in hot spots for say a year or two. With data from the pilot studies we can tell if the the vaccine has made an impact or not.
If and when the vaccine is approved, the public must not be complacent and forgo public health measures which need to be maintained. The war on dengue can be waged on many fronts and we would be foolhardy not to utilise all means available.
For doctors, there are ongoing discussions and debates on dengue in the Dobbs Facebook Group (Malaysia’s largest doctors only group in Facebook with over 10,000 members). Malaysian doctors can ask to join but please look out for a verification request message in your Facebook inbox as the group is for doctors only
